Q: Electrons Orbit around the nucleus of an atom...
i just want to see where this goes
EDIT: heres an image

by orbit i mean electrons love around the nucleus like shown in the above image.....like a planet around a sun....

they vibrate? i learnt that cuz electrons move so fast, it's impossible to actually know what their "orbits" are like. the orbitals are just predictions of where the electrons would most likely be.mike2005 wrote:exactly. they dont orbit, they vibrate. back in forth in differente wavelengths depending on how 'excited' they are and in what shell.


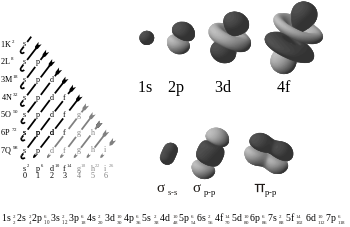


XemnasXD wrote:yeah you guys basically got it after mike said something. In high school i was taught that Electrons orbited around the nucleus and when we learned about orbitals it was never explained that the orbitals weren't just areas of orbit. I wonder why school teaches you stuff like that, most ppl go most of their school life getting wrong info and it really doesn't help in preparing you for higher educations....


takolin wrote:I voted false.
Highschool says that they orbit....
[i]blah blah blah, science thingy, blah blah blah donuts, blah blah image blah...

Key-J wrote:takolin wrote:I voted false.
Highschool says that they orbit....
[i]blah blah blah, science thingy, blah blah blah donuts, blah blah image blah...
Highschool? Didn't you guys get taught about like Zee most basic atomic structures back in Middle school?
O, and yeah. With the basic defenition you can say that indeed they do orbit the Nucleus of the Atom, however, as mentioned before. At higher lvls you will learn that they don't actually orbit in fixed orbitals, it all depends on different factors.
Then of course you have Ionic and Coavalent bonds, Electron sharing, Electron Transfer, Electronegativity, and other kind's of Bonds. Van Der Walls forces n blah blah...