Page 1 of 1
Simple Question
Posted: Tue Nov 06, 2007 6:02 am
by XemnasXD
I just want to see what your education system has taught you...
Q: Electrons Orbit around the nucleus of an atom...
i just want to see where this goes
EDIT: heres an image

by orbit i mean electrons love around the nucleus like shown in the above image.....like a planet around a sun....
Posted: Tue Nov 06, 2007 6:11 am
by Reise
Electrons orbit around my wang.
Posted: Tue Nov 06, 2007 6:14 am
by XemnasXD
fair enough
with an e-penis that large you probably do have orbits, congratz to you sir
Posted: Tue Nov 06, 2007 6:18 am
by TOloseGT
lol, random question.
Posted: Tue Nov 06, 2007 6:22 am
by XemnasXD
yeah its random but i want to see how ppl will respond so plz even if you don't post participate in the poll...
Posted: Tue Nov 06, 2007 6:41 am
by Deathsythe
Yes electrons orbit the nucleus. I'm pretty sure about this since i passed the AP chem test
Posted: Tue Nov 06, 2007 7:17 am
by mike2007
i said false.
Posted: Tue Nov 06, 2007 7:36 am
by TOloseGT
by ur definition of orbit, i'd say false. there are different electron orbitals and each orbital is a different "shape".
Posted: Tue Nov 06, 2007 7:39 am
by mike2007
TOloseGT wrote:by ur definition of orbit, i'd say false. there are different electron orbitals and each orbital is a different "shape".
exactly. they dont orbit, they vibrate. back in forth in differente wavelengths depending on how 'excited' they are and in what shell.
Posted: Tue Nov 06, 2007 7:43 am
by FX
when i charge up my nuke are those the electrons and protons around me???
and when a glaiver kills me, where are my neutrons

Posted: Tue Nov 06, 2007 8:18 am
by TOloseGT
mike2005 wrote:exactly. they dont orbit, they vibrate. back in forth in differente wavelengths depending on how 'excited' they are and in what shell.
they vibrate? i learnt that cuz electrons move so fast, it's impossible to actually know what their "orbits" are like. the orbitals are just predictions of where the electrons would most likely be.
Posted: Tue Nov 06, 2007 8:36 am
by Sylhana
I said false, but to not affect the poll, I shall refrain from answering 
Posted: Tue Nov 06, 2007 9:20 am
by Innovacious
I got a BB (double award) in science when i was in school. Cant remember jack shit now though. Science is of no importance to me, its all been pushed out of my brain to make way for more important info.
Posted: Tue Nov 06, 2007 9:28 am
by takolin
I voted false.
Highschool says that they orbit, but later you'll learn that they linger somewhere near the nucleus.
And you can't define the place and the speed of an electron at the same time, thus we assume that they have a 99% (iirc) chance off being in a defined area called an orbital.
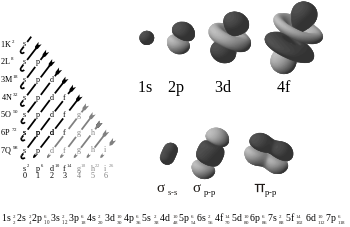
But it's been too long to give a nice explanation unless I go check my books of my 1st year in college.
Posted: Tue Nov 06, 2007 9:42 am
by Sylhana
^takolin got it :/ If I'm not mistaken, shrodinger's equation calculates the highest chance where they will be, thus the donuts you see in the pic is a visual representation of the electron distribution.
Posted: Tue Nov 06, 2007 3:23 pm
by XemnasXD
yeah you guys basically got it after mike said something. In high school i was taught that Electrons orbited around the nucleus and when we learned about orbitals it was never explained that the orbitals weren't just areas of orbit. I wonder why school teaches you stuff like that, most ppl go most of their school life getting wrong info and it really doesn't help in preparing you for higher educations....
@deathsythe - o rly?
but yeah its like my said the vibrate back and forth up down whatever whenever depending on the spin of the electron and the other electrons around it...orbitals just mention the area where they are most likely to be found theoretically....
Posted: Tue Nov 06, 2007 3:31 pm
by takolin
XemnasXD wrote:yeah you guys basically got it after mike said something. In high school i was taught that Electrons orbited around the nucleus and when we learned about orbitals it was never explained that the orbitals weren't just areas of orbit. I wonder why school teaches you stuff like that, most ppl go most of their school life getting wrong info and it really doesn't help in preparing you for higher educations....
Because orbital theory isn't the most easiest thing of chem and unless you don't study something scientific, it won't really matter.
But the line thing isn't really wrong for S orbitals because they have the highest chance of being there in the orbital.
Posted: Tue Nov 06, 2007 3:34 pm
by XemnasXD
not really even for the S orbitals just because something has the highest chance of being with that spherical shell it doesn't mean that the electrons have to orbit around the nucleus to do so....theoretically if an atom is in the S orbitals it could very well decide to stay on only 1 half of the S orbital shell, theres no way to tell if its doing it but you see what i mean...
Posted: Tue Nov 06, 2007 3:43 pm
by Key-J
takolin wrote:I voted false.
Highschool says that they orbit....
[i]blah blah blah, science thingy, blah blah blah donuts, blah blah image blah...
Highschool? Didn't you guys get taught about like Zee most basic atomic structures back in Middle school?
O, and yeah. With the basic defenition you can say that indeed they do orbit the Nucleus of the Atom, however, as mentioned before. At higher lvls you will learn that they don't actually orbit in fixed orbitals, it all depends on different factors.
Then of course you have Ionic and Coavalent bonds, Electron sharing, Electron Transfer, Electronegativity, and other kind's of Bonds. Van Der Walls forces n blah blah...
Posted: Tue Nov 06, 2007 3:48 pm
by XemnasXD
I learned about Orbitals in my senior year of high school and im pretty sure it was only because i was in the highest chem course our school offered at the highest lvl and that was only Chem II honors...
Posted: Tue Nov 06, 2007 5:23 pm
by takolin
Key-J wrote:takolin wrote:I voted false.
Highschool says that they orbit....
[i]blah blah blah, science thingy, blah blah blah donuts, blah blah image blah...
Highschool? Didn't you guys get taught about like Zee most basic atomic structures back in Middle school?
O, and yeah. With the basic defenition you can say that indeed they do orbit the Nucleus of the Atom, however, as mentioned before. At higher lvls you will learn that they don't actually orbit in fixed orbitals, it all depends on different factors.
Then of course you have Ionic and Coavalent bonds, Electron sharing, Electron Transfer, Electronegativity, and other kind's of Bonds. Van Der Walls forces n blah blah...
WTH is middle school?
Primary school
High or secondary school
College
^all I know.
Sure I learned about orbitals in my last year, but in general people don't (I followed a scientific direction).
Posted: Tue Nov 06, 2007 5:38 pm
by .//curb
4+4=8?


